Chapter One General Principles
The first article is to ensure that the drug clinical trial process is standardized, the results are scientific and reliable, and the rights and interests of subjects are protected and their safety is guaranteed. In accordance with the "Drug Administration Law of the People's Republic of China" and the "Regulations for the Implementation of the Drug Administration Law of the People's Republic of China", this specification is formulated with reference to internationally recognized principles.
Article 2 Drug clinical trial quality management practices are the standard requirements for the entire clinical trial process, including plan design, organization and implementation, monitoring, auditing, recording, analysis, summary, and reporting.
Article 3 All phases of clinical trials, human bioavailability or bioequivalence trials must be implemented in accordance with this specification.
Article 4 All human-targeted research must comply with the "World Medical Congress Helsinki Declaration" (Appendix 1), that is, fairness, respect for personality, and strive to maximize the benefits of subjects and avoid harm as much as possible.
Chapter Two "Pre-Clinical Trial Preparations and Necessary Conditions
Article 5: Drug clinical trials must have sufficient scientific basis. Before conducting a human test, the purpose of the test and the problems to be solved must be carefully considered, and the expected benefits and risks to the health of the subjects and the public should be weighed, and the expected benefits should exceed the possible damage. The selection of clinical trial methods must comply with scientific and ethical requirements.
Article 6: Drugs for clinical trials shall be prepared and provided by the sponsor. Before conducting a clinical trial, the sponsor must provide pre-clinical research data of the trial drug, including prescription composition, manufacturing process, and quality inspection results. The pre-clinical information provided must meet the requirements for conducting the corresponding phases of clinical trials, and at the same time, the effectiveness and safety information related to the clinical trials that have been completed and the clinical trials in other regions should be provided. The preparation of clinical trial drugs shall comply with the "Good Manufacturing Practice for Drugs".
Article 7: The facilities and conditions of drug clinical trial institutions shall meet the needs of safe and effective clinical trials. All investigators should have the professional expertise, qualifications and abilities to undertake the clinical trial, and be trained. Before the start of a clinical trial, the investigator and the sponsor should reach a written agreement on the trial plan, trial supervision, inspection and standard operating procedures, as well as the division of responsibilities in the trial.
Chapter III Protection of the Rights and Interests of Subjects
Article 8 In the process of drug clinical trials, the personal rights and interests of subjects must be given Adequate guarantee, and ensure the scientificity and reliability of the test. The rights, safety and health of subjects must be higher than consideration of scientific and social interests. The ethics committee and informed consent are the main measures to protect the rights and interests of subjects.
Article 9 To ensure the rights and interests of subjects in clinical trials, an independent ethics committee must be established and filed with the State Food and Drug Administration. The ethics committee should be composed of at least five people who are engaged in medical-related professionals, non-medical professionals, legal experts and personnel from other units, and have members of different genders. The composition and work of the ethics committee should not be affected by any participants in the experiment.
Article 10 The trial protocol must be reviewed and approved by the ethics committee and signed and approved before it can be implemented. During the trial period, any modification of the trial protocol should be approved by the ethics committee; serious adverse events during the trial should be reported to the ethics committee in time.
Article 11: The review opinions of the ethics committee on the clinical trial protocol shall be decided by voting after discussion, and the members participating in the clinical trial shall be evaded. Experts who are not committee members can be invited to attend the meeting due to work needs, but they will not vote. The ethics committee should establish working procedures, all meetings and their resolutions should have written records, and the records should be kept for five years after the end of the clinical trial.
Article 12 The ethics committee shall strictly review the trial protocol in accordance with the following items from the perspective of protecting the rights and interests of subjects:
(1) Qualification, experience, and adequacy of the investigator Time to participate in the clinical trial, whether the staffing and equipment conditions meet the trial requirements;
(2) Whether the trial plan fully considers the ethical principles, including the purpose of the research, the risks that the subjects and other personnel may suffer And the benefits and the scientific nature of the trial design;
(3) The method of selection of subjects, and whether the subjects (or their family members, guardians, legal representatives) are provided with complete information about the trial It is easy to understand, whether the method of obtaining informed consent is appropriate;
(4) Treatment and/or insurance measures given when subjects are harmed or even died due to participation in clinical trials;
(5) Whether the proposed amendments to the trial protocol are acceptable;
(6) Periodically review the risk level of subjects in clinical trials.
Article 13: After receiving the application, the ethics committee shall hold a meeting in time, review and discuss, issue written opinions, and attach the list of members attending the meeting, professional status and my signature. The opinion of the ethics committee can be:
(1) agree;
(2) agree after making necessary amendments;
(3) disagree;< /p>
(4) Terminate or suspend approved trials.
Article 14 The investigator or its designated representative must explain the details of the clinical trial to the subject:
(1) The subject’s participation in the trial should be voluntary , And have the right to withdraw from the trial at any time at any stage of the trial without being discriminated against or retaliated, and their medical treatment and rights will not be affected;
(2) Subjects must be made aware of and participate in the trial And the personal information in the trial is confidential. When necessary, the drug regulatory authority, ethics committee or sponsor can consult the data of subjects participating in the trial according to regulations;
(3) Trial purpose, trial process and time limit, inspection operation, test subject The participants anticipate possible benefits and risks, and inform the participants that they may be assigned to different groups of the trial;
(4) Subjects must be given sufficient time to consider whether they are willing to participate in the trial. Subjects who express their consent should provide the above introduction and explanation to their legal representatives. The informed consent process should adopt the language and writing that the subject or legal representative can understand. During the trial, the subject can know the relevant information at any time;
(5) If there is any trial-related In the event of damage, the subject can receive treatment and corresponding compensation.
Article 15 The informed consent form shall be obtained after a full and detailed explanation of the experiment:
(1) The subject or his legal representative shall sign the informed consent form And indicate the date, the researcher performing the informed consent process also needs to sign the name and date on the informed consent form;
(2) For subjects with incompetence, if the ethics committee agrees in principle, When the researcher believes that the subjects’ participation in the trial is in their own interests, these patients can also enter the trial, and at the same time they should obtain the consent of their legal guardian, sign and date; (3) children as subjects If the child is involved, he must obtain the informed consent of his legal guardian and sign an informed consent form. When the child can make a decision to participate in the study, he must also obtain his own consent;
(4) In an emergency If the informed consent form of the person and his legal representative cannot be obtained, if there is a lack of proven effective treatment methods, and the experimental drug is expected to save lives, restore health, or relieve pain, it can be considered as a subject, but it needs to be tested The protocol and related documents clearly state the method of accepting these subjects, and obtain the prior consent of the ethics committee;
(5) If important new information related to the trial drug is found, the informed consent form must be revised in writing After the approval of the ethics committee, the subject's consent was obtained again.
Chapter IV Trial Protocol
Article 16 A trial protocol should be formulated before the start of a clinical trial, and the protocol should be mutually agreed between the investigator and the sponsor Sign it and report to the ethics committee for approval before implementation.
Article 17 The clinical trial plan should include the following content:
(1) Trial title;
(2) Trial purpose, trial background, preclinical The clinically significant discoveries in the research and the clinical trial results related to the trial, the known possible risks and benefits to the human body, and the possibility of ethnic differences in the trial drugs;
(3) Sponsor’s Name and address, the place where the experiment was conducted, the name, qualification and address of the investigator;
(4) The type of experiment design, the randomization grouping method and the level of blinding;
(5) Subjects’ inclusion criteria, exclusion criteria, and rejection criteria, steps to select subjects, and method of allocation of subjects; (6) Calculate based on statistical principles to achieve the intended purpose of the test The number of cases required;
(7) The dosage form, dosage, route of administration, method of administration, number of administrations, course of treatment, and regulations on combined drugs, as well as regulations on packaging and labeling of test drugs Description;
(8) The items to be clinically and laboratory checked, the number of determinations and pharmacokinetic analysis, etc.;
(9) Registration and use of experimental drugs Recording, delivery, distribution methods and storage conditions;
(10) Clinical observation, follow-up and measures to ensure compliance of subjects;
(11) Criteria for suspending clinical trials , The provisions of the end of clinical trials;
(12) Efficacy evaluation criteria, including the method of evaluation parameters, observation time, recording and analysis;
(13) Subject’s Procedures for the preservation of codes, random number tables and case report forms;
(14) Adverse event recording requirements and serious adverse event reporting methods, treatment measures, follow-up methods, time and outcome;< /p>
(15) Establishment and preservation of experimental drug codes, methods of unblinding and regulations on unblinding in emergency situations;
(16) Statistical analysis plan, statistical analysis data set The definition and selection of data;
(17) Data management and data traceability regulations;
(18) Quality control and quality assurance of clinical trials;
(19) Trial-related ethics;
(20) Expected progress and completion date of clinical trials;
(21) Follow-up after trial And medical measures;
(twenty-two) the responsibilities and other relevant regulations of the parties;
(twenty-three) references.
Article 18: In clinical trials, if necessary, the trial protocol can be amended according to the prescribed procedures.
Chapter 5 Responsibilities of Investigators
Article 19 Investigators in charge of clinical trials should meet the following requirements:
(1) Have the corresponding professional technical positions and medical qualifications in medical institutions;
(2) Have the professional knowledge and experience required in the experimental plan;
(3) Right The clinical trial method has rich experience or can obtain the academic guidance of the experienced researcher of the unit;
(4) Be familiar with the clinical trial-related materials and literature provided by the sponsor;
(5) The right to control the personnel participating in the test and the equipment required for the use of the test.
Article 20 Researchers must read and understand the contents of the trial protocol in detail, and strictly follow the protocol.
Article 21 Investigators should understand and be familiar with the nature, effect, efficacy and safety of the trial drug (including relevant data of the drug’s preclinical research), and should also grasp the findings during the clinical trial All new information related to the drug.
Article 22 Researchers must conduct clinical trials in a medical institution with good medical facilities, laboratory equipment, and staffing. The institution shall have all the facilities for handling emergencies to ensure that subjects Security. The laboratory test results should be accurate and reliable.
Article 23 The investigator shall obtain the consent of the medical institution or the competent unit to ensure that there is sufficient time to be responsible for and complete the clinical trial within the time limit specified in the plan. The researcher must explain the relevant trial information, regulations and responsibilities to all staff participating in the clinical trial, and ensure that there are sufficient numbers of subjects who meet the trial protocol to enter the clinical trial.
Article 24 The investigator shall explain to the subject the detailed information about the trial approved by the ethics committee, and obtain an informed consent.
Article 25: Investigators are responsible for making medical decisions related to clinical trials and ensuring that subjects receive appropriate treatment when adverse events occur during the trial.
Article 26 Researchers are obliged to take necessary measures to ensure the safety of subjects and record them. If a serious adverse event occurs during the clinical trial, the investigator should immediately take appropriate treatment measures to the subject, and at the same time report to the drug supervision and management department, the health administration department, the sponsor and the ethics committee, and sign and indicate on the report Date.
Article 27 Researchers shall ensure that the data are truthful, accurate, complete, timely, and legally included in the medical records and case report forms.
Article 28 Researchers shall accept the inspections and inspections of the inspectors or inspectors dispatched by the sponsor and the inspections and inspections of the drug supervision and administration department to ensure the quality of clinical trials.
Article 29 The investigator and the sponsor shall negotiate the cost of the clinical trial and specify it in the contract. In the process of clinical trials, the researcher shall not charge subjects for the fees required for the trial medication.
Article 30: After the completion of the clinical trial, the investigator must write a summary report, sign and indicate the date before sending it to the sponsor.
Article 31: Investigators must notify subjects, sponsors, ethics committees, and drug regulatory authorities to suspend a clinical trial, and explain the reasons.
Chapter VI Sponsor’s Responsibilities
Article 32 The sponsor is responsible for initiating, applying, organizing, monitoring and auditing a clinical trial , And provide experimental funding. Sponsors submit applications for clinical trials to the State Food and Drug Administration in accordance with relevant national laws and regulations, and may also entrust contract research organizations to perform certain tasks and tasks in clinical trials.
Article 33 The sponsor selects the institution and investigator of the clinical trial, and recognizes its qualifications and conditions to ensure the completion of the trial.
Article 34 The sponsor shall provide an investigator’s handbook, which includes the chemistry, pharmacy, toxicology, pharmacology and clinical (including previous and ongoing trials) information and data of the experimental drug .
Article 35 Sponsors can organize clinical trials according to the protocol only after obtaining the approval of the State Food and Drug Administration and obtaining the approval of the ethics committee.
Article 36: Sponsors and investigators jointly design clinical trial plans, stating the responsibilities and division of labor in terms of plan implementation, data management, statistical analysis, result reporting, and publication methods. Sign the experimental plan and contract agreed by both parties.
Article 37 The sponsor shall provide investigators with easily identifiable, correctly coded and specially labeled test drugs, standard products, control drugs or placebos, and ensure that the quality is qualified. Test drugs should be appropriately packaged and stored according to the needs of the test plan. The sponsor should establish a management system and a record system for experimental drugs.
Article 38: The sponsor shall appoint qualified inspectors and be accepted by the investigator.
Article 39 The sponsor shall establish a quality control and quality assurance system for clinical trials, and can organize audits of clinical trials to ensure quality.
Article 40 The sponsor should quickly investigate the serious adverse events that occur with the investigator, take necessary measures to ensure the safety and rights of the subjects, and promptly report to the drug supervision and management department and the health administration. Department reports, and at the same time notify other investigators involved in clinical trials of the same drug.
Article 41 The sponsor must notify the investigator, the ethics committee and the State Food and Drug Administration before suspending a clinical trial, and state the reason.
Article 42: The sponsor is responsible for submitting the summary report of the trial to the State Food and Drug Administration.
Article 43 The sponsor shall provide insurance for subjects participating in clinical trials, and bear the cost of treatment and corresponding financial compensation for subjects who have suffered trial-related damage or death. The sponsor shall provide the researcher with legal and economic guarantees, except for those caused by medical malpractice.
Article 44 When the investigator does not follow the approved protocol or relevant regulations to conduct a clinical trial, the sponsor should point out for correction. If the situation is serious or persists, the investigator should be terminated. Clinical trials and report to the drug supervision and administration department.
Chapter 7 Duties of Inspectors
Article 45 The purpose of inspections is to ensure that the rights and interests of subjects in clinical trials are protected. Ensure that the data in the test records and reports are accurate, complete and correct, and ensure that the test complies with the approved plan and relevant laws and regulations.
Article 46 The inspector is the main contact between the sponsor and the researcher. The number and the number of visits depend on the complexity of the clinical trial and the number of medical institutions participating in the trial. Inspectors should have appropriate medical, pharmacy or related professional qualifications, and have undergone necessary training to be familiar with the relevant regulations of drug management, familiar with the preclinical and clinical information about the trial drug, as well as the clinical trial protocol and related documents.
Article 47 Inspectors shall follow standard operating procedures and supervise the progress of clinical trials to ensure that clinical trials are carried out according to the plan. The specific content includes:
(1) Before the test, confirm that the test undertaker has the appropriate conditions, including staffing and training, the laboratory is well-equipped and functioning well, and has various tests related to the test Conditions, it is estimated that there will be a sufficient number of subjects, and the participating researchers are familiar with the requirements of the trial protocol;
(2) During the trial, monitor the investigator’s implementation of the trial protocol, and confirm that before the trial Obtain informed consent of all subjects, understand the selection rate of subjects and the progress of the trial, and confirm that the selected subjects are qualified;
(3) Confirm that all data records and reports are correct and complete , All case report forms are filled in correctly and consistent with the original data. All errors or omissions have been corrected or noted, signed and dated by the investigator. The dosage changes, treatment changes, combined medications, intermittent diseases, loss to follow-up, and missed inspections of each subject should be confirmed and recorded. Verify that the withdrawal and loss of the selected subjects have been explained in the case report form;
(4) Confirm that all adverse events are recorded, and that serious adverse events are reported within the specified time and recorded in
(5) Verify that the experimental drugs are supplied, stored, distributed, and retracted in accordance with relevant laws and regulations, and make corresponding records;
(6) Assist the investigator to carry out the necessary Notification and application matters, and report trial data and results to the sponsor;
(7) The follow-up that the investigator failed to do, the trial that was not conducted, the inspection that was not conducted, and whether it was correct should be clearly and truthfully recorded. Errors and omissions shall be corrected;
(8) A written report shall be sent to the sponsor after each visit. The report shall state the date and time of the inspection, the name of the inspector, and the findings of the inspection.
Chapter 8 "Records and Reports
Article 48 "Medical records, as the original documents of clinical trials, should be kept intact. The data in the case report form comes from the original document and is consistent with the original document. Any observations and inspection results in the experiment should be recorded in the medical record in a timely, accurate, complete, standardized, and truthful manner and correctly filled in the case report form, and should not be arbitrarily If the change is due to a mistake, the original record should be kept clear and defensible when making any corrections, and the corrector should sign the name and time.
Article 49 Various laboratory data in clinical trials should be recorded or a copy of the original report should be pasted on the case report form, and data within the normal range should also be recorded in detail. Data that deviates significantly or are outside the clinically acceptable range must be verified. The test item must indicate the unit of measurement used.
Article 50: To protect the privacy of subjects, the subject’s name should not appear on the case report form. The researcher should confirm the identity of the subject according to the code of the subject and record it.
Article 51 The content of the clinical trial summary report should be consistent with the requirements of the trial protocol, including:
(1) The actual number of cases randomly entered into each group, and the cases dropped and excluded And the reasons;
(2) Comparison of baseline characteristics between different groups to determine comparability;
(3) Statistical analysis and clinical significance analysis of all efficacy evaluation indicators. The interpretation of statistical results should focus on its clinical significance;
(4) Safety evaluation should include clinical adverse events and reasonable statistical analysis of laboratory indicators, and detailed description and evaluation of serious adverse events;
p>
(5) To evaluate the efficacy of a multi-center trial, the differences between centers and their impact should be considered;
(6) The effect and safety of the trial drug and the relationship between risk and benefit A brief overview and discussion of the relationship.
Article 52 The data in clinical trials must be preserved (Appendix 2) and managed in accordance with regulations. The investigator shall keep the clinical trial data until five years after the termination of the clinical trial. The sponsor shall keep the clinical trial data until five years after the trial drug is approved for marketing.
Chapter 9 Data Management and Statistical Analysis
Article 53 The purpose of data management is to quickly, completely and accurately include test data in the report , All the various steps involved in data management must be recorded in order to check the quality of the data and the implementation of the experiment. Use appropriate procedures to ensure the confidentiality of the database, and there should be computer database maintenance and support procedures.
Article 54 The allocation of subjects in clinical trials must be carried out according to the random allocation plan determined by the trial design, and the treatment group code of each subject should be used as a blind base by the sponsor and the investigator. Save. The blinding test shall specify the conditions for unblinding and the procedures for performing unblinding in the plan, and be accompanied by emergency letters with corresponding processing codes. In emergencies, it is allowed to break blindness of individual subjects and understand the treatment they receive, but the reasons must be stated in the case report form.
Article 55 The statistical analysis process of clinical trial data and the expression of its results must adopt standardized statistical methods. Biostatistics professionals are required to participate in all stages of clinical trials. The clinical trial plan needs to have a statistical analysis plan, which should be confirmed and refined before the formal statistical analysis. If an interim analysis is required, the reasons and operating procedures should be explained. The evaluation of the effect of treatment should consider the confidence interval and the result of the hypothesis test together. The selected statistical analysis data set needs to be explained. The missing, unused or redundant information must be explained, and the statistical report of the clinical trial must be consistent with the summary report of the clinical trial.
Chapter Ten "Management of Experimental Drugs
Article 56 "Clinical trial drugs shall not be sold.
Article 57: The sponsor shall be responsible for the proper packaging and labeling of drugs used in clinical trials, and marking them as specific for clinical trials. In double-blind clinical trials, the test drug and the control drug or placebo should be consistent in appearance, smell, packaging, labeling, and other characteristics.
Article 58 The use records of experimental drugs shall include information on quantity, shipment, delivery, acceptance, distribution, recovery and destruction of remaining drugs after application, etc.
Article 59 The use of experimental drugs is the responsibility of the investigator. The investigator must ensure that all experimental drugs are only used for subjects in the clinical trial, and their dosage and usage should follow the trial protocol. The remaining experimental drugs shall be returned to the sponsor, and the above process shall be responsible and recorded by a special person, and the experimental drugs shall be managed by a special person. Researchers are not allowed to transfer experimental drugs to any non-clinical trial participants.
Article 60 The supply, use, storage of experimental drugs and the handling of remaining drugs shall be inspected by relevant personnel.
Chapter 11 Quality Assurance
Article 61 Sponsors and investigators shall perform their respective duties and strictly follow the clinical trial protocol. Standard operating procedures are adopted to ensure the quality control of clinical trials and the implementation of quality assurance systems.
Article 62 All observations and findings related to clinical trials should be verified, and quality control must be carried out at each stage of data processing to ensure that the data is complete, accurate, true and reliable.
Article 63 The drug regulatory authority and sponsor may entrust inspectors to conduct systematic inspections of clinical trial related activities and documents to evaluate whether the trial complies with the trial plan, standard operating procedures and relevant regulatory requirements If the test data is recorded in a timely, true, accurate and complete manner. The audit should be performed by personnel who are not directly involved in the clinical trial.
Article 64 The drug supervision and administration department shall inspect the respective tasks and implementation status of the investigator and the sponsor in the implementation of the trial. The relevant materials and documents (including medical records) of medical institutions and laboratories participating in clinical trials should be inspected by the drug regulatory authority.
Chapter 12 Multi-center Trials
Article 65 Multi-center trials are conducted by multiple investigators in different locations according to the same trial protocol. Clinical trials conducted by the unit at the same time. Each center starts and ends the experiment at the same time. The multi-center trial is headed by one principal investigator, who acts as the coordinating investigator among the centers of the clinical trial.
Article 66 The planning, organization and implementation of multi-center trials should consider the following points:
(1) The trial plan shall be discussed by the main investigator of each center and the sponsor. Confirmation, implementation after the approval of the ethics committee;
(2) At the beginning of the clinical trial and during the middle period, a researcher meeting should be organized;
(3) Each center conducts clinical trials at the same time;
(4) The sample size of clinical trials in each center and the distribution between centers should meet the requirements of statistical analysis;
(5) Ensure that the same procedures are used to manage experimental drugs in different centers, Including distribution and storage;
(6) Train researchers participating in the experiment according to the same experiment plan;
(7) Establish standardized evaluation methods and the laboratory used in the experiment Both clinical evaluation methods and clinical evaluation methods should have unified quality control, and laboratory inspections can also be carried out by a central laboratory; (8) Data information should be centrally managed and analyzed, and data transmission, management, verification and query should be established Procedure;
(9) To ensure that the investigators of each trial center comply with the trial protocol, including terminating their participation in the trial if they violate the protocol.
Article 67: Multi-center trials should establish a management system based on the number of centers participating in the trial and the requirements of the trial, as well as the level of understanding of the trial drugs, and coordinate the investigator to be responsible for the implementation of the entire trial.
Chapter 13 Supplementary Provisions
Article 68 The meaning of the following terms in this specification is:
Clinical Trial ), refers to any systematic study of drugs in humans (patients or healthy volunteers) to confirm or reveal the effects, adverse reactions and/or absorption, distribution, metabolism and excretion of the test drug, with the purpose of determining the test drug The efficacy and safety.
The test protocol (Protocol) describes the background, theoretical basis and purpose of the test, test design, method and organization, including statistical considerations, test execution and completion conditions. The plan must be signed and dated by the main investigator, research institution and sponsor participating in the trial.
Investigator's Handbook (Investigator?, sBrochure), is the clinical and non-clinical research data of the relevant experimental drugs in human research.
Informed Consent (InformedConsent) refers to the subject informing all aspects of a trial, and the subject voluntarily confirms that he agrees to participate in the clinical trial process. It must be signed and dated The informed consent form serves as documentary proof.
Informed Consent Form (InformedConsentForm) is a documentary evidence that each subject expresses their willingness to participate in a trial. The researcher needs to explain to the subjects the nature of the trial, the purpose of the trial, the possible benefits and risks, other available treatment methods, and the rights and obligations of the subjects in accordance with the Declaration of Helsinki, so that the subjects fully understand After expressing his consent.
The Ethics Committee (EthicsCommittee) is an independent organization composed of medical professionals, legal experts, and non-medical personnel. The safety, health and rights of the subjects are protected. The composition and all activities of the committee should not be interfered or influenced by the clinical trial organization and implementer.
Investigator, the person who conducts clinical trials and is responsible for the quality of clinical trials and the safety and rights of subjects. Researchers must undergo a qualification review and have the professional expertise, qualifications and abilities for clinical trials.
Coordinating Investigator (Coordinating Investigator), an investigator who is responsible for coordinating the work of investigators in each center in a multi-center clinical trial.
Sponsor, a company, institution or organization that initiates a clinical trial and is responsible for the trial’s initiation, management, finance, and monitoring.
Monitor, a person with relevant knowledge appointed by the sponsor and responsible for the sponsor, whose task is to monitor and report the progress of the test and verify data.
Audit refers to a systematic inspection conducted by personnel who are not directly involved in the test to evaluate whether the implementation of the test, the recording and analysis of data are consistent with the test protocol, standard operating procedures, and drugs Compliant with relevant regulatory requirements for clinical trials.
Inspection: The drug regulatory authority conducts an official review of the relevant documents, facilities, records and other aspects of a clinical trial. The inspection can be conducted at the location of the trial unit, the sponsor or the location of the contract research organization .
Case Report Form (CaseReportForm, CRF), refers to a document designed according to the test protocol, used to record the data of each subject during the test.
Investigational Products, used for investigational drugs, control drugs or placebos in clinical trials.
Adverse Event (AdverseEvent), an adverse medical event that occurs after a patient or clinical trial subject receives a drug, but it does not necessarily have a causal relationship with the treatment.
Serious Adverse Event, an event that requires hospitalization, prolonged hospitalization, disability, impact on work ability, life-threatening or death, and congenital malformations occurred during the clinical trial.
Standard Operating Procedure (SOP) is a standard and detailed written procedure for the effective implementation and completion of each task in a clinical trial.
Blinding/Masking is a procedure in which one or more parties do not know the treatment allocation of subjects in a clinical trial. Single-blind means that the subject does not know the subject, and double-blind means that none of the subjects, investigators, monitors, or data analysts know the treatment allocation.
Contract Research Organization (ContractResearchOrganization, CRO), an academic or commercial scientific institution. The sponsor may entrust it to perform certain tasks and tasks in the clinical trial, and such entrustment must be stipulated in writing.
Article 69: The State Food and Drug Administration is responsible for the interpretation of this specification.
Article 70 This specification shall come into effect on September 1, 2003, and the former “Regulations for the Administration of Drug Clinical Trials” issued by the State Drug Administration on September 1, 1999 shall be repealed at the same time.
Appendix 1:
The Helsinki Declaration of the World Medical Congress
The Ethical Code of Human Medical Research
Adopted: the 18th World Medical Congress , Helsinki, Finland. June 1964
Revision: The 29th World Medical Congress, Tokyo, Japan, October 1975
The 35th World Medical Congress, Venice, Italy, October 1983
The 41st World Medical Congress, Hong Kong, September 1989
The 48th World Medical Congress, South Africa, October 1996
The 52nd World Medical Congress, Edinburgh, Scotland, October 2000
I. Introduction
1. The Declaration of Helsinki drafted by the World Medical Congress is a statement of the ethical guidelines for human medical research to guide doctors and other participants in human medical research. Human medical research includes research on the human body itself and related data or information.
2. It is the duty of doctors to promote and protect human health. The knowledge and ethics of doctors are precisely to perform this duty.
3. The Geneva Declaration of the World Medical Congress restricts doctors with the language "The health of the patient must be our first consideration." The international standard of medical ethics declares: "Only when it is in the interests of the patient, the doctor can provide medical measures that may adversely affect the patient's physiology and psychology."
4. The advancement of medicine is based on research, which to a certain extent ultimately depends on experiments with human subjects.
5. In human medical research, consideration of subjects’ health should take precedence over scientific and social interests.
6. The main purpose of human medical research is to improve prevention, diagnosis and treatment methods, and to improve the understanding of disease etiology and pathogenesis. Even the best prevention, diagnosis and treatment methods that have been proven should be continuously tested to test their effectiveness, efficiency, feasibility and quality through research.
7. In medical practice and medical research, most prevention, diagnosis and treatment involve risks and burdens.
8. Medical research should comply with ethical standards, respect all people and protect their health and rights. Some subjects are vulnerable groups and need special protection. The special needs of the economically and medically disadvantaged must be recognized. Particular attention should be paid to those subjects who cannot give or refuse informed consent, subjects who may only give informed consent under duress, subjects who do not benefit from the research, and those who receive treatment at the same time. Examiner.
9. Researchers must be aware of the ethical, legal and regulatory requirements for human research in their country, and must comply with international requirements. The ethics, laws and regulations of any country do not allow the reduction or cancellation of the protection provided for subjects in this declaration.
2. Basic principles of medical research
10. In medical research, it is the responsibility of doctors to protect the life and health of subjects and maintain their privacy and dignity.
11. Human medical research must comply with generally accepted scientific principles, and be based on a comprehensive understanding of scientific literature and related materials, and sufficient laboratory and animal tests (if necessary).
12. Research that may affect the environment must be carried out with due care, and the rights of laboratory animals used for research must be respected.
13. The design and implementation of each human trial should be clearly stated in the trial plan, and the trial plan should be submitted to the ethics approval committee for review, comment, guidance, and review and approval under appropriate circumstances. The ethics committee must be independent of the investigator and sponsor, and not be affected by any other aspects. The ethics committee should abide by the laws and regulations of the country where the experiment is conducted. The committee has the authority to supervise ongoing trials. Researchers are responsible for submitting monitoring data to the committee, especially data on all serious adverse events.研究人员还应向委员会提交其他资料以备审批,包括有关资金、申办者、研究机构以及其它对受试者潜在的利益冲突或鼓励的资料。
14.研究方案必须有关于伦理方面的考虑的说明,并表明该方案符合本宣言中所陈述的原则。
15.人体医学研究只能由有专业资格的人员并在临床医学专家的指导监督下进行。必须始终是医学上有资格的人员对受试者负责,而决不是由受试者本人负责,即使受试者已经知情同意参加该项研究。
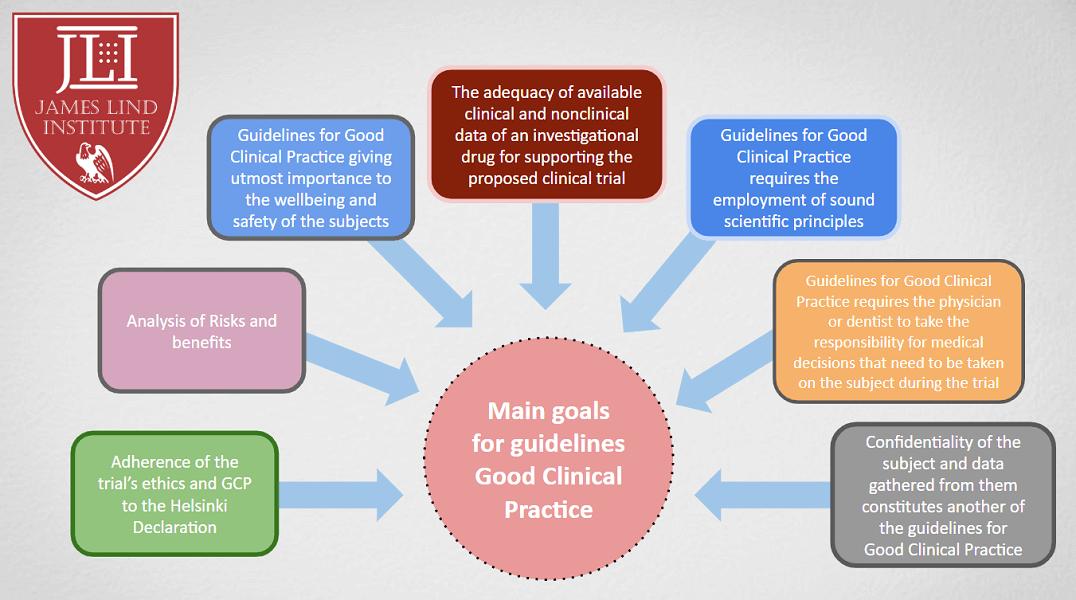
16.每项人体医学研究开始之前,应首先认真评价受试者或其他人员的预期风险、负担与受益比。这并不排除健康受试者参加医学研究。所有研究设计都应公开可以获得。
17.医生只有当确信能够充分地预见试验中的风险并能够较好地处理的时候才能进行该项人体研究。如果发现风险超过可能的受益或已经得出阳性的结论和有利的结果时医生应当停止研究。
18.人体医学研究只有试验目的的重要性超过了受试者本身的风险和负担时才可进行。这对受试者是健康志愿者时尤为重要。
19.医学研究只有在受试人群能够从研究的结果中受益时才能进行。
20.受试者必须是自愿参加并且对研究项目有充分的了解。
21.必须始终尊重受试者保护自身的权利。尽可能采取措施以尊重受试者的隐私、病人资料的保密并将对受试者身体和精神以及人格的影响减至最小。
22.在任何人体研究中都应向每位受试侯选者充分地告知研究的目的、方法、资金来源、可能的利益冲突、研究者所在的研究附属机构、研究的预期的受益和潜在的风险以及可能出现的不适。应告知受试者有权拒绝参加试验或在任何时间退出试验并且不会受到任何报复。当确认受试者理解了这些信息后,医生应获得受试者自愿给出的知情同意,以书面形式为宜。如果不能得到书面的同意书,则必须正规记录非书面同意的获得过程并要有见证。
23.在取得研究项目的知情同意时,应特别注意受试者与医生是否存在依赖性关系或可能被迫同意参加。在这种情况下,知情同意的获得应由充分了解但不参加此研究与并受试者也完全无依赖关系的医生来进行。
24.对于在法律上没有资格,身体或精神状况不允许给出知情同意,或未成年人的研究受试者,研究者必须遵照相关法律,从其法定全权代表处获得知情同意。只有该研究对促进他们所代表的群体的健康存在必需的意义,或不能在法律上有资格的人群中进行时,这些人才能被纳入研究。
25.当无法定资格的受试者,如未成年儿童,实际上能作出参加研究的决定时,研究者除得到法定授权代表人的同意,还必须征得本人的同意。
26.有些研究不能从受试者处得到同意,包括委托人或先前的同意,只有当受试者身体/精神状况不允许获得知情同意是这个人群的必要特征时,这项研究才可进行。应当在试验方案中阐明致使参加研究的受试者不能作出知情同意的特殊原因,并提交伦理委员会审查和批准。方案中还需说明在继续的研究中应尽快从受试者本人或法定授权代理人处得到知情同意。
27.作者和出版商都要承担伦理责任。在发表研究结果时,研究者有责任保证结果的准确性。与阳性结果一样,阴性结果也应发表或以其它方式公之于众。出版物中应说明资金来源、研究附属机构和任何可能的利益冲突。与本宣言中公布的原则不符的研究报告不能被接受与发表。
三、医学研究与医疗相结合的附加原则
28.医生可以将医学研究与医疗措施相结合,但仅限于该研究已被证实具有潜在的预防、诊断和治疗价值的情况下。当医学研究与医疗措施相结合时,病人作为研究的受试者要有附加条例加以保护。
29.新方法的益处、风险、负担和有效性都应当与现有最佳的预防、诊断和治疗方法作对比。这并不排除在没有有效的预防、诊断和治疗方法存在的研究中,使用安慰剂或无治疗作为对照。
30.在研究结束时,每个入组病人都应当确保得到经该研究证实的最有效的预防、诊断和治疗方法。
31.医生应当充分告知病人其接受的治疗中的那一部分与研究有关。病人拒绝参加研究绝不应该影响该病人与医生的关系。
32.在对病人的治疗中,对于没有已被证明的预防、诊断和治疗方法,或在使用无效的情况下,若医生判定一种未经证实或新的预防、诊断和治疗方法有望挽救生命、恢复健康和减轻痛苦,在获得病人的知情同意的前提下,应不受限制地应用这种方法。在可能的情况下,这些方法应被作为研究对象,并有计划地评价其安全性和有效性。记录从所有相关病例中得到的新资料,适当时予以发表。同时要遵循本宣言的其他相关原则。
附录2:
临床试验保存文件
一、临床试验准备阶段
临床试验保存文件 | 研究者 | 申办者 | |
1 | 研究者手册 | 保存 | 保存 |
2 | 试验方案及其修正案(已签名) | 保存原件 | 保存 |
3 | 病例报告表(样表) | 保存 | 保存 |
4 | 知情同意书 | 保存原件 | 保存 |
5 | 财务规定 | 保存 | 保存 |
6 | 多方协议(已签名)(研究者、申办者、合同研究组织) | 保存 | 保存 |
7 | 伦理委员会批件 | 保存原件 | 保存 |
8 | 伦理委员会成员表 | 保存原件 | 保存 |
9 | 临床试验申请表 | 保存原件 | |
10 | 临床前实验室资料 | 保存原件< /p> | |
11 | 国家食品药品监督管理局批件 | 保存原件 | |
12 | 研究者履历及相关文件 | 保存 | 保存原件 |
13 | 临床试验有关的实验室检测正常值范围 | 保存 | 保存 |
14 | 医学或实验室操作的质控证明 | 保存原件 | 保存 |
15 | 试验用药品的标签 | 保存原件 | |
临床试验保存文件 | 研究者 | 申办者 | |
16 | 试验用药品与试验相关物资的运货单 | 保存 | 保存 |
17 | 试验药物的药检证明 | 保存原件 | |
18 | 设盲试验的破盲规程 | 保存原件 | |
19 | 总随机表 | 保存原件 | |
20 | 监查报告 | 保存原件 |
二、临床试验进行阶段
| 临床试验保存文件 | 研究者 | 申办者 | |
21 | 研究者手册更新件 | 保存 | 保存 |
22 | 其他文件(方案、病例报告表、知情同意书、书面情况通知)的更新 | 保存 | 保存 |
23 | 新研究者的履历 | 保存 | 保存原件 |
24 | 医学、实验室检查的正常值范围更新 | 保存 | 保存 |
25 | 试验用药品与试验相关物资的运货单 | 保存 | 保存 |
26 | 新批号试验药物的药检证明 | 保存原件 | |
27 | 监查员访视报告 | 保存原件 | |
28 | 已签名的知情同意书 | 保存原件 | |
29 | 原始医疗文件 | 保存原件 | |
30 | 病例报告表(已填写,签名,注明日期) | 保存副本 | 保存原件 |
31 | 研究者致申办者的严重不良事件报告 | 保存原件 | 保存 |
临床试验保存文件 | 研究者 | 申办者 | |
32 | 申办者致药品监督管理局、伦理委员会的严重不良事件报告 | 保存 | 保存原件 |
33 | 中期或年度报告 | 保存 | 保存 |
34 | 受试者鉴认代码表 | 保存原件 | |
35 | 受试者筛选表与入选表 | 保存 | 保存 |
36 | 试验用药品登记表 | 保存 | 保存 |
37 | 研究者签名样张 | 保存 | 保存 |
三、临床试验完成后
临床试验保存文件 | 研究者 | 申办者 | |
38 | 试验药物销毁证明 | 保存 | 保存 |
39 | 完成试验受试者编码目录 | 保存 | 保存 |
40 | 稽查证明件 | 保存原件 | |
41 | 最终监查报告 | 保存原件 | |
42 | 治疗分配与破盲证明 | 保存原件 | |
43 | 试验完成报告(致伦理委员会国家食品药品监督管理局) | 保存原件 | |
44 | 总结报告 | 保存 | 保存原件 |
